Introduction
Microfluidic devices operate with small amounts of samples through micrometer and/or sub-micrometer channels1. As the main characteristics, these devices show reduced size, low weight, and high throughput. These characteristics have attracted tremendous and widespread attention by the synchrotron community2,3 interested in compatible instrumentation for in situ and in vivo experiments. To date, reported applications of these devices on synchrotrons, involving studies on protein crystallography4,5, advanced materials6, electrochemistry7,8, catalysis9,10,11, in vivo experiments (eukaryotic and bacteria cells)6,12,13, established a paramount experimental contribution for drawing a picture of these complexes systems, providing precious information about electronic, structural, and chemical properties in real-time, in situ and/or operando. However, developing a small, versatile, and compact microfluidic device able to operate in synchrotron beamlines, especially at nanoprobes where the X-ray beam size is only hundreds of nanometers, has remained a challenge due to sample environment requirements. Arguably, limits should be established on such devices. It is crucial to define what is the good compromise between the selected material and the reliability of the microfabrication process when the device is to be applied in experiments that cover a wide window of the electromagnetic spectrum (X-rays, Infrared or visible light)14.
In the last two decades, poly(dimethylsiloxane), commonly called PDMS (an elastomer), has been the most applied material as substrate and sealing layers in microfluidic devices15,16,17 due to its remarkable physical and chemical properties such as biocompatibility, flexibility, transparency, and low toxicity1,18,19. Nevertheless, PDMS presents some disadvantages that should be considered: it is permeable to small molecules due to its porosity, which can affect the devices throughput20, and it shows low chemical resistance to many organic solvents21. Hence, this elastomer can lead to non-accurate results in biological assays and it is restricted to analyses in aqueous media. Similar limitations are also found in poly(methyl methacrylate) (PMMA), another popular polymer substrate for the production of microfluidic chips. In addition, PDMS devices might reach millimeters of thickness, which is non-compatible with X-rays measurements due to the lower transmission coefficient for X-rays when compared to commonly applied polymers in microfluidic. For example, a millimeter thick cyclic olefin copolymer (COC) based device attenuates, at 12.4 keV, seven-fold less than PDMS22, being more discrepant at lower energies. A similar proportion is observed by comparing polyester to PDMS.
An insightful approach to minimize the drawbacks mentioned before, consists in drastically changing the sealing process by selecting a sealing layer transparent to X-rays and chemically resistant, which leads to well known polymer films such as polyester, polypropylene, polyamide, and polycarbonate (Supporting Information). In addition, few works have proposed using ultraviolet (UV) sensitive adhesive, like the Norland Optics Adhesive (NOA), as substrate or adhesion layer23,24,25,26 in microfluidic devices. NOA is a thiolene based polymer that attracted attention due to exhibiting interesting properties like low cost, optical transparency in the visible range, high chemical resistance to strong solvents (acetone, hydrochloric acid, toluene)24,27, and hydrophobicity26 becoming a great candidate for applications in synchrotron beamlines28,29.
Motivated by the relevance and impact of synchrotron studies for diverse areas as well as by the challenges in manufacturing microfluidic devices for such applications, we have developed the microfabrication of a three-electrode microfluidic device based on a new sealing method, which is compatible with X-rays (in reflection mode), infrared, and visible light. The device has been designed with only two main parts; a glass substrate, and a thin polymer employed as a sealing layer. We have used a UV-sensitive adhesive to promote adherence between polyester and glass. It is worth noting that the microfabrication involves well-stablished and scalable techniques and the resulting device presents satisfactory bonding strength and high chemical stability in both organic and biological media. The approach adopted in this work converged to a multifunctional microfluidic device.
Results and discussions
Microfabrication procedure
Initially, 3 cm ×× 3 cm glass squares were cut, cleaned up using piranha solution at a temperature of 60 ∘∘ C, and then used as a substrate. The microchannels were prepared in a clean room using a geometry defined by the photolithography mask. In total, four lithography masks or channel geometry, were prepared: 100 𝜇μm (C1) and 100 𝜇μm plus a reservoir (res) with 300 𝜇μm (C2), 200 𝜇μm (C3), and 200 𝜇μm plus a reservoir(res) with 600 𝜇μm (C4). Prior to the microchannel patterning, a layer of 60 nm of chromium was evaporated on the clean glass, working as a protective layer that improves the isotropic corrosion of the glass. To prepare the channels, the trenches were exposed to a wet chemical etching30 using a solution at HF 49%, NH4F 1.38 M, and HCl 38%, and hydrofluoric acid (HF) 20%, resulting in smooth channels. After the glass corrosion, using a second photolithography mask, three electrodes were patterned, and then gold was deposited on the channels by magnetron sputtering. After the deposition of the three gold electrodes, the device was drilled to enable connecting the inlet and outlet tubes. The sequence of the channel and electrode preparation is shown in Fig. S1 (Supporting Information).
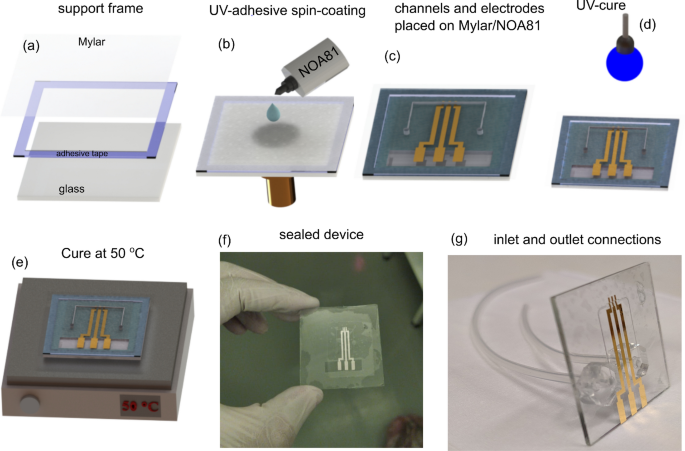
The most important and delicate step of this work is the sealing process, once the sealing layer is a thin film. To succeed, an adhesive composed of NOA and a polyester film was prepared, herein this stack is also called sticker, which simplifies the sealing process. The sealing steps are summarized in Fig. 1. Firstly, a polyester film with 12 𝜇μm of thickness was fixed with double-face adhesive tape on a glass frame to ensure mechanical stability (Fig. 1a). Thus, the stack glass-frame/polyester was transferred to the spin-coater and a thin film (up to 1 μμm) of NOA was prepared on the polyester film at 5000 rpm for 35 s. Then, the device was attached to the NOA side as shown in Fig. 1b–c. Finally, a cure was done exposing the device to ultraviolet light (𝜆λ=365 nm) for 2 min at room temperature. After the curing step, the device was kept at 50 ∘∘ C for 12 h to improve the adhesion (Fig. 1d,e). In order to access the three gold electrodes, the spare piece of the polyester film was cut-off from the sealed device (Fig. 1f). In the last step, the glass was drilled in the backside, and the inlet and outlet connections (silicone tubes) were welded using PDMS support, which gives high mechanical stability to the connections. All the sealing steps were carried out into a cleanroom.
Sequence of the sealing process (a) Glass frame used as a support for the polyester film (b–c) Spin-coating process of the NOA on the polyester film, and the procedure to attach the device to the “stikcker” (d–e) UV-cure (2 min) and the thermal annealing at 50 ∘∘ C (12 h) employed to improve the adhesion between the device and sealing layer (f–g) Shows the device completely sealed, and after bound the inlet and outlet connections, respectively.

generic 5mg cialis
generic 5mg cialis