Abstract
Knowing how biomarker levels vary within biological fluids over time can produce valuable insight into tissue physiology and pathology, and could inform personalised clinical treatment. We describe here a wearable sensor for monitoring biomolecule levels that combines continuous fluid sampling with in situ analysis using wet-chemical assays (with the specific assay interchangeable depending on the target biomolecule). The microfluidic device employs a droplet flow regime to maximise the temporal response of the device, using a screw-driven push-pull peristaltic micropump to robustly produce nanolitre-sized droplets. The fully integrated sensor is contained within a small (palm-sized) footprint, is fully autonomous, and features high measurement frequency (a measurement every few seconds) meaning deviations from steady-state levels are quickly detected. We demonstrate how the sensor can track perturbed glucose and lactate levels in dermal tissue with results in close agreement with standard off-line analysis and consistent with changes in peripheral blood levels.
Introduction
Chemical signalling changes continuously in the human body. Biomarker concentrations rise and fall in complex patterns and on different time scales: from hourly to daily fluctuations of metabolites, hormones and inflammatory changes, to millisecond spiking of ions and neurotransmitters at neuronal synapses1,2,3. Continuous measurement of these dynamic chemical signals has significant implications for fundamental physiological science, and can play a crucial role in the development of disease diagnostics, therapeutics, treatments and new drugs4.
Recent years have seen the development of continuous wearable technologies for non-invasive monitoring (of sweat, tears, etc.)5,6,7,8,9, however, there are questions as to how representative these measurements are of underlying tissue and blood levels. Implanted electrochemical sensors can directly measure tissue biochemistry and have the potential to provide high temporal resolution10,11, but foreign body response12 and performance drift due to long-term stability of bio-recognition sites (such as immobilised proteins and antibodies) pose great challenges to sensor viability13,14. An alternative approach is to develop miniaturised analytical devices based on microfluidics.
Microfluidics allows the miniaturisation of laboratory assays and detection technology to create micro total analysis systems, which have been successfully applied to point-of-care (POC) diagnostics15,16,17,18. Current POC devices (e.g., lateral flow devices18) focus on single-measurement procedures. While accurate and user-friendly, they are manually intensive and the inherent low measurement frequency limits the ability to track dynamic chemical changes. Hence, the application of microfluidics to continuous monitoring requires a shift of strategy to autonomous systems that integrate technology for sampling, liquid handling and analysis. This poses challenges as microfluidic systems typically rely on bulky laboratory equipment such as syringe pumps, external valves and microscopes—technology ill-suited for wearable devices.
Here, we present a fully integrated wearable microfluidic sensor, which not only provides accurate, precise and robust fluidic sampling and control, but also in situ chemical assaying using droplets as microreactors. It addresses the challenges of POC monitoring by providing accurate real-time continuous measurement with high temporal resolution in a small wearable package. We demonstrate and validate the device in vivo by using it to monitor perturbed glucose and lactate levels in dermal tissue in healthy volunteers.
Results
Device operation
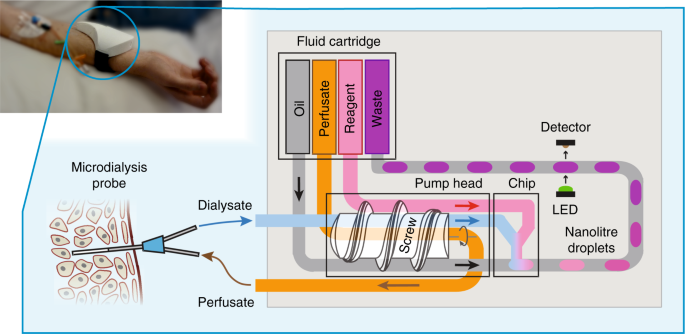
The sensor combines a miniature peristaltic pump, microfluidic chip, optical flow cell, electronics and a fluid reservoir cartridge—all integrated into a small wearable package (Fig. 1, top left), and employs a droplet-flow regime which gives optimal temporal response in microfluidic analysis systems19,20,21,22. The fluidic architecture can be tailored depending on the analyte and the requirements of its assay. Figure 1 shows the simplest fluidic setup where analysis is via a simple mix-and-read colorimetric assay. The sensor connects to a microdialysis probe (composed of concentrically arranged inlet and outlet tubes connected by a permeable membrane at the probe tip) to allow sampling of interstitial fluid by flowing a sterile perfusate solution (such as phosphate buffered saline, PBS) through the probe. Molecules can freely diffuse from the interstitial fluid into the perfusate at the probe tip, yielding a clean dialysate that is directly representative of the chemical composition of the interstitial fluid. The dialysate is pulled into the sensor, through the pump and then on into a microfluidic chip where it meets an analyte-specific reagent and is subsequently broken into a stream of droplets by introduction of an immiscible oil. Within each droplet the dialysate mixes and reacts with the reagent to generate a coloured product. The concentration of the target analyte determines the strength of the droplet’s colour, which is quantified by an inline optical flow cell. The data from the flow cell is passed to an on-board microcontroller which relays it to an external data-processing device using an integrated Bluetooth transmitter, and also saves it to an SD card.